Many sourcing teams face delays, unclear certifications, and inconsistent mask quality. This article highlights the top disposable face mask manufacturers so you can identify dependable partners instantly. With this list, you avoid guesswork and quickly find factories that meet international requirements and stable production needs.
1. 3M Company-Disposable Face Mask Manufacturer in the USA
3M, founded in 1902 as Minnesota Mining and Manufacturing Company, became a global leader in respiratory protection by the 1950s. The company has advanced filter materials and an N95 design over the decades. During the 2020 COVID-19 pandemic, the U.S. Department of Defense contracted 3M to produce billions of disposable face masks, with hospitals, first responders, and industries relying on trusted models like 1860, 8210, and 1870+.
Filter Technology and Patent Portfolio
3M developed melt-blown filtration and electrostatic charging methods. These techniques power modern N95 performance. The company’s processes deliver filtration efficiency above 95% while keeping breathing resistance low. Core models like the 3M 8210 and 3M 1860 pass NIOSH 42 CFR Part 84 testing every time.
Medical-grade versions meet ASTM F2100 standards. You get bacterial filtration efficiency (BFE ≥98%), particulate filtration efficiency (PFE ≥98%), and resistance to synthetic blood. The 1860s and 1870+ respirators work in operating rooms and intensive care units. These areas have the highest fluid splash risk.
Product Lines Across Use Cases
– Medical N95 Respirators: Models 1860, 1860S, and 1870+ carry NIOSH N95 certification and FDA 510(k) clearance for surgical use
– Industrial N95 Series: The 8210, 8210V (with exhalation valve), and 8511 block construction dust, metalworking fumes, and non-oil particles
– Surgical & Procedure Masks: Triple-layer disposable masks rated ASTM Level 1–3. They meet BFE/PFE standards and pressure specs for routine clinical work
– FFP2/FFP3 for Europe: CE-marked respirators meet EN 149 standards. EU healthcare and industrial sites use them often
Regulatory Standing and Market Position
3M holds FDA 510(k) clearance for several surgical respirator models. NIOSH re-audits production sites to check Part 84 compliance. In Europe, FFP-rated products carry CE marking under the Personal Protective Equipment Regulation.
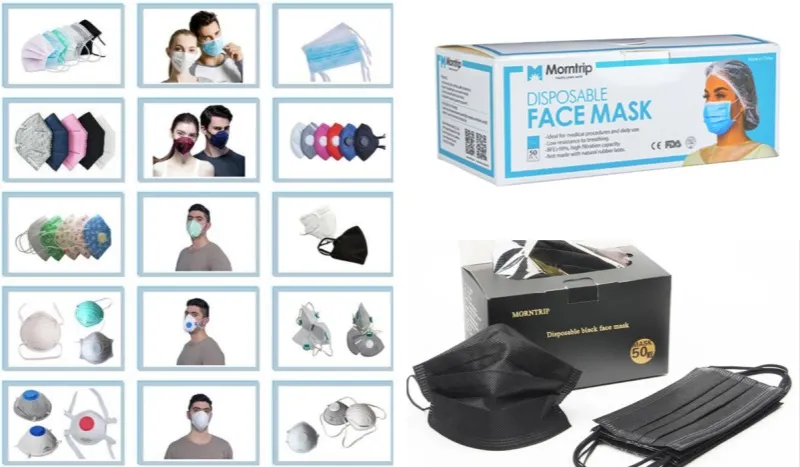
2. Morntrip-Disposable Face Mask Manufacturer in China
Wuhan Morntrip Trading Co., Ltd. runs one of China’s most integrated PPE production chains. We make nonwoven fabrics in-house and assemble masks with automation—all under one roof. Our 50,000-square-meter facility has 251-500 workers. About 80% of our production lines run on automation. This gives you tighter quality control. Plus, bulk orders get faster response times.
Production Capacity at Scale
We produce 30 million disposable flat masks per month. On top of that, you get 5 million dust and particulate respirator masks. We also make 600,000 disposable coveralls and 1 million isolation gowns each month. Hospitals, government agencies, and chain distributors can source all their PPE from one supplier. No need to split orders across multiple vendors. This makes customs clearance simpler. Container loading gets easier, too.
Integration as a Competitive Edge
Most disposable face mask manufacturers buy materials from outside suppliers. We don’t. Morntrip produces its own PP and SMS nonwoven fabrics on-site. Raw material shortages happen. Our self-sufficiency cuts lead times by 5-7 days. Active orders get priority. We handle the full workflow—from meltblown filtration layers to finished surgical masks. Supply chain problems remain minimal. Filter performance stays consistent across batches.
Compliance and Global Reach
Our products ship to over 120 countries. Documentation meets EN, ASTM, and ISO standards. We hold ISO 9001 quality management certification. Product-specific test reports cover CE/EN and ASTM F standards. We’ve served many markets. Our team handles different labeling rules, testing protocols, and regulatory paperwork for Europe, North America, and emerging markets.
Key Advantages for B2B Buyers
– One-Stop PPE Sourcing : Get masks, coveralls, and isolation gowns from one certified PPE supplier
– High Automation : 80% automated processes keep ear-loop tension stable, ultrasonic sealing clean, and dimensions accurate
– Raw Material Control : In-house nonwoven production cuts dependency and speeds up order turnaround
– Volume Flexibility : Our capacity handles mid-sized hospital networks and national government tenders
3. Honeywell International Inc.-Disposable Face Mask Manufacturer in the USA
Honeywell runs factories in the U.S., Mexico, China, and India. The company sends industrial safety gear to over 150 countries. You’ll find NIOSH-approved N95 masks, KN95 versions for China, and FFP2/FFP3 models with EN 149 certification for Europe.
Two Production Lines: Industrial and Medical
Honeywell makes two different mask types. The DC365 series works for construction, mining, and factory settings. These masks block non-oil particles—dust, metal fumes, pollen—at over 95% filtration. The DF300 series has FDA 510(k) clearance. It meets ASTM F2100 Level 2 and Level 3 standards. You get fluid resistance ≥120 mmHg, BFE ≥98%, and PFE ≥98%. Hospitals use DF300 units in operating rooms and emergency areas with high blood splash risk.
Certifications by Region
– NIOSH 42 CFR Part 84 : All N95 models pass particle filtration tests with TC approval numbers
– FDA 510(k) Clearance : Medical-grade DF300 respirators work for surgical use
– CE Marking (EN 149) : FFP2 /FFP3 respirators meet European PPE Regulation 2016/425
– ISO 9001 & ISO 13485 : Quality systems cover factories and medical device production
Built for Long Shifts
Honeywell masks have soft inner layers, adjustable nose bridges, and shaped cups. The RWS-54008 model folds flat. Tuck it in your pocket. Workers wear it for 8-hour shifts without marks. Exhalation valves on some models cut heat by 30%. Easier breathing means workers keep masks on longer.
Fast Delivery Network
Honeywell’s global reach means local stock hubs. North American buyers get products from Rhode Island warehouses. European orders ship from Germany. Bulk orders arrive in 7–10 days. Government agencies and large companies want suppliers with global logistics. Honeywell handles regular orders and sudden demand spikes.
Procurement directors running cross-border operations trust Honeywell’s wide certification range and reliable delivery.
4. Kimberly-Clark Corporation-Disposable Face Mask Manufacturer in the USA
Kimberly-Clark, founded in 1872 in Wisconsin, built well-known consumer brands like Kleenex, Huggies, and Kotex before entering medical-grade PPE. By the 2010s, North American hospitals relied on their surgical masks, gowns, and drapes. The Halyard Health division operated until 2014, after which Kimberly-Clark continued producing procedural masks and protective apparel under Kimberly-Clark Professional.
Medical-Grade Product Range
We make ASTM F2100 Level 1–3 surgical masks . You get bacterial filtration efficiency (BFE) ≥95–98% and particulate filtration efficiency (PFE) ≥95–98%. Fluid resistance reaches ≥120 mmHg on Level 2 models and ≥160 mmHg on Level 3 units. These specs meet operating room and ICU standards. The mask lineup includes tie-on, ear-loop, and integrated-visor designs. Some models use latex-free materials. They skip fiberglass parts to reduce allergy risk.
We also supply surgical gowns rated AAMI PB70 Level 1–4, isolation gowns, sterile gloves (latex and nitrile), and surgical drapes. Hospitals can order complete surgical kits—mask, gown, gloves, drapes—from one supplier. This makes inventory tracking easier. Vendor audits get simpler, too.
Certifications and Compliance
– FDA Regulation : Many products are Class II medical devices. Some have 510(k) clearance; others qualify for exemptions
– CE Marking : European models meet MDR requirements and EN 14683 ( medical masks ) or EN 13795 (surgical gowns and drapes)
– ISO 13485 : Our manufacturing sites follow quality management systems. We track each batch and control sterile assurance levels (SAL)
– ASTM & AAMI Standards : Products pass standard tests for barrier performance, breathability, and synthetic blood penetration
Hospital Procurement Advantage
We work with group purchasing organizations (GPOs) and large hospital systems in North America. Long-term contracts lock in pricing and delivery schedules. Procurement teams like this stability. You skip spot-market swings. Brand recognition speeds up approval during hospital reviews. Clinicians trust names they’ve used for years.
5. Moldex-Metric-Disposable Face Mask Manufacturer in the USA
Moldex-Metric started in 1980 in Culver City, California. We built our name on molded respirator technology and foam earplugs. Today, we run factories in the U.S. and Europe. Our products ship to over 50 countries. Revenue sits between $50 million and $150 million USD per year. Staff numbers range from 180 to 750 employees. This depends on production peaks.
Patent-Driven Respirator Design
We hold more than 50 patents for respirators and hearing protection. The EZ-ON harness system lets workers put on and take off N95 masks in seconds. The HandyStrap design hangs the respirator around your neck between uses. You don’t need to touch the filter surface. The Dura-Mesh outer shell holds its shape in high humidity. Industrial crews and hospital staff get better seal performance during long shifts.
NIOSH and FDA Clearances
All our filtering facepiece respirators carry NIOSH approvals under 42 CFR Part 84. You get TC-84A approval numbers for N95 , N99, N100, R95, and P100 models. The 1500 and 1700 series hold FDA surgical N95 clearance. These work in operating rooms or ICUs. They meet fluid resistance and filtration standards for medical settings.
The 2200, 2300, 2400, 2500, 2700, and 2800 series suit industrial sites. Some models add carbon layers. These block low levels of ozone or organic vapors. Construction, mining, oil and gas, and manufacturing buyers choose these. They protect against dust, fumes, and particles.
Reusable Respirators
We make half-mask and full-face reusable respirators in the 7000, 7800, and 9000 series. Bayonet-style cartridge connections work with NIOSH-approved filters. These handle particles, organic vapors, acid gases, and mixed gases. Paint booths, chemical handling, or asbestos removal facilities use these. They save money per use.
ISO 9001 Quality and COVID-19 Scale-Up
Our plants hold ISO 9001 certification through Underwriters Laboratories. Batch tracking and process controls meet medical device and safety standards. During COVID-19, we added shifts and hired extra staff. Production hit near-maximum capacity. We kept healthcare workers and first responders stocked. This proved we can scale fast. Governments and hospitals can count on us for volume.
6. Prestige Ameritech-Disposable Face Mask Manufacturer in the USA
Prestige Ameritech runs the largest U.S. surgical mask factory right in Texas. They have built medical gear at home since 2005. The 2020 pandemic hit, and American hospitals needed to stop relying on imports. They made Prestige their top choice. Premier Inc. and 15 major health systems even invested in the company. They signed a 6-year deal to lock in a steady mask supply.
100% Made in the USA
What makes them different? They sell 100% of their products to U.S. customers. They don’t export a thing. This helps safety managers significantly. You remove the risk of foreign trade bans or shipping delays. Other supply chains broke down during COVID-19. Prestige Ameritech kept running. Today, buying teams keep them on contract. This gives you a reliable local backup.
Product Line: Medical-Grade Focus
– ProGear Surgical Masks : Ear-loop and tie-on models rated ASTM Level 1–3. Fluid resistance hits ≥160 mmHg on top units
– N95 Respirators : NIOSH-approved filters for hospital use
– Face Shields and Disposable PPE : Extra protective gear for operating rooms and isolation wards
– OEM Manufacturing : Custom production for other medical brands under private label
FDA Compliance and Hospital Approvals
Surgical masks fall under FDA Class II medical device rules. Prestige Ameritech holds FDA registrations and 510(k) clearances for hospital-grade masks . Premier’s investment and multi-year contracts show the company passed tough quality checks. Large hospital systems sign long-term deals with suppliers that meet ISO 13485-aligned quality systems, batch tracking, and sterile checks.
7. Winner Medical Group-Disposable Face Mask Manufacturer in China
Winner Medical Co., Ltd., listed on the Shenzhen Stock Exchange (ticker 300888.SZ), was founded in 1991 and went public in 2020. During the COVID-19 pandemic, revenue surged to CNY 12.53 billion in 2020, and by mid-2025, annual revenue reached around CNY 9.67–10.8 billion, about 25% year-on-year growth. The company operates production bases across Hubei, Jiangsu, and other Chinese provinces, employing over 15,600 staff globally. With major investments in non-woven fabric mills and mask production lines, Winner Medical maintains high-volume manufacturing to meet domestic and international demand efficiently.
3-Ply Surgical Mask Portfolio
Our core line includes 3-ply ear-loop and tie-on masks. They meet China YY 0469-2011, ASTM, and EN 14683 Type II/IIR standards. You get:
– Bacterial filtration efficiency (BFE) ≥95–98%
– Particulate filtration efficiency (PFE) ≥95%
– Fluid resistance ≥120–160 mmHg on Type IIR models
– Differential pressure (ΔP) within breathability limits
We use spunbond–meltblown–spunbond (SMS/SMMS) structures. The middle layer runs 20–25 g/m² meltblown PP. Outer layers are 20–25 g/m² spunbond PP. Nose clips adjust easily. Ultrasonic ear-loop welding keeps size consistent batch after batch.
Product Range by Use Case
– Sterile surgical masks: EO-sterilized units for operating rooms
– Non-sterile medical masks: Outpatient clinics, wards, retail channels
– Pediatric sizes: Smaller dimensions for children
– Printed variants: Designs with color patterns for retail
– High-protection masks: Type IIR splash resistance for surgical use
CE, FDA, and Export Compliance
Winner Medical holds CE marking under EU MDR for Class I medical devices and PPE categories. Test reports cover EN 14683 (surgical masks) and EN 149 (respirators). We also offer FDA-registered masks for the U.S. market. Our team has experience with wound dressings, surgical drapes, and PPE. This track record speeds up paperwork for new export markets.
8. Shanghai Dasheng Health Products Manufacturing Disposable Face Mask Manufacturer in China
Shanghai Dasheng Health Products Manufacture Co., Ltd. makes N95 respirators and surgical masks at its Shanghai factory. They use multi-layer non-woven and melt-blown fabric to reach ≥95% filtration on respirators. Industry reports put revenue around USD 500 million. They hold about 5% market share in the Asia-Pacific N95 and surgical mask segments. Public financial data varies by source. Both numbers come from third-party estimates, not audited reports.
Dual Product Strategy: Medical and Industrial
Dasheng operates two separate production lines. Medical-grade disposable masks use non-woven layers with bacterial filtration (BFE) ≥95–98%. Hospitals and clinics stock these for routine use. Industrial N95 respirators serve construction, mining, and factory sites. Workers use them to block dust, fumes, and fine particles on the job. Market reports rank Dasheng among major N95 makers for construction, oil & gas, and factory safety programs.
NIOSH Approval and U.S. Regulatory Changes
Dasheng models once carried NIOSH approvals under 42 CFR Part 84. They passed filtration tests (≥95% NaCl aerosol). They also met breathing resistance standards. In August 2021, the U.S. FDA and NIOSH pulled authorization for all Shanghai Dasheng respirators. U.S. healthcare and workplace buyers had to stop using those units. Earlier certifications and test data no longer met acceptance rules. Dasheng can still sell to markets outside the U.S., under NIOSH control. But U.S. buyers must check the current approval status before ordering.
Export Reach and Global Listings
Global N95 market reports list Dasheng next to major international brands. This shows they export to many countries. Models come with English packaging, NIOSH codes (where valid), and batch tracking for overseas buyers. Emergency supply lists featured Dasheng units during the first pandemic waves. Buyers outside the U.S. can purchase these respirators if their local rules accept non-NIOSH certifications. They can also buy if Dasheng gets new approvals.
Managers buying in Asia-Pacific or checking Chinese N95 suppliers? Confirm current certification status and local compliance before signing contracts.
9. Owens & Minor-Disposable Face Mask Manufacturer in the USA
Owens & Minor, Inc. (NYSE: OMI) connects medical distribution and PPE manufacturing. They built a nationwide logistics network over 110 years. The company started in Richmond, Virginia, in 1915. It grew from a regional supplier into a traded healthcare giant. Peak revenue topped $9 billion. The workforce reached over 20,000 employees. Today, they run dozens of regional distribution centers. Their just-in-time (JIT) warehouse systems serve thousands of U.S. hospitals, surgery centers, and clinics.
Surgical Mask Production and Pandemic Response
We make ASTM Level 1–3 surgical masks , procedure masks, and NIOSH-approved N95 respirators. Our production facilities span the Americas and Asia. They handle melt-blown filtration layers, non-woven fabric forming, and final assembly steps. COVID-19 changed everything. The U.S. government named us a critical domestic and nearshore PPE source. Hospital buyers got priority access to masks, gowns, and gloves. This mattered most during import delays.
Distribution Network as a Competitive Edge
We stock hundreds of thousands of SKUs. You’ll find our own brands (HALYARD, O&M) plus third-party lines. Regional hubs run vendor-managed inventory (VMI) programs. Hospitals get same-day or next-day delivery. We also offer cost transparency reports and clinical department standardization programs. These services cut inventory turnover days. Stockouts drop too. Group purchasing organization (GPO) contracts lock in multi-year pricing. Large health systems benefit most.
Procurement directors across U.S. hospital networks count on us. They need integrated supply chain services. Our mask manufacturing track record speaks for itself. Volume contract stability seals the deal.
10. GAMA Healthcare -Disposable Face Mask Manufacturer in the UK
GAMA Healthcare Ltd. takes a different path with PPE. They focus on infection prevention as a complete system, not separate products. Started in 2004, the company operates from Watford, Hertfordshire. They serve hospitals across the UK, Europe, and growing markets.
Most companies just make masks. GAMA bundles surgical masks , disinfecting wipes, surface sanitizers, and portable isolation units into infection control packages. Procurement teams get one supplier for multiple critical items. This simplifies hospital-wide safety.
Bundled Solutions for Hospital Buyers
GAMA’s Clinell brand leads UK hospital surface cleaning. The company pairs Clinell wipes with surgical masks, hand sanitizers, and training materials. Bundling cuts down on vendor management work. Hospitals need fewer contracts. Invoices come together faster.
The Rediroom portable isolation ward sets up in hours. It uses negative-pressure airflow. This keeps sick patients separate during outbreaks. Rediair air purifiers and Violet UV room cleaning units round out the options. ICU or infectious disease ward managers get full solutions from one certified supplier.
Infection Control Expertise as a Differentiator
GAMA employs clinical educators and infection control experts. They train hospital teams on mask use, surface cleaning, and PPE procedures. Most mask sellers ship and disappear. GAMA stays through setup. This cuts down on mistakes. Compliance rates go up. Long NHS partnerships prove it works at scale.
Hospital buyers looking for infection prevention programs—not just boxes of masks—find value here. GAMA’s system approach and UK healthcare record deliver results.